The ethylene oxide sterilizers manufacturers must be flexible and certify a high level of quality both for the facility and process. During every project steps and especially for design and manufacturing, it has to be demonstrated that any validation and qualification aspects should be taken into account from the very beginning until the project finalization.
Ethylene oxide sterilization validation and qualification
The qualification and validation of an ethylene oxide facility are essential to ensure quality, safety, reliability and efficiency of the process. For RSD, it is an important part of our work in which we invest time and high-precision work to ensure an optimum result.
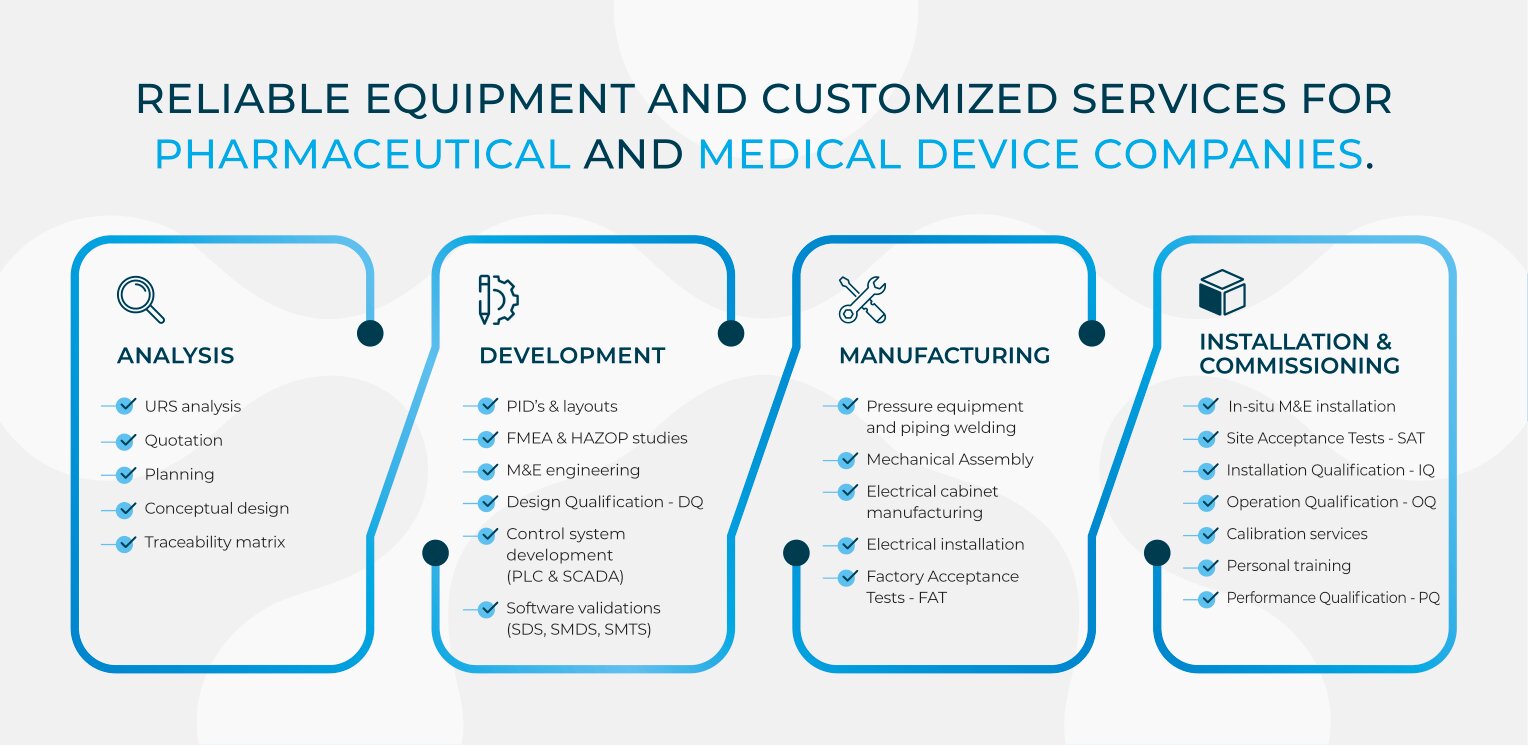
To do so, we use to follow the GMP guidelines. They should be incorporated quality in the design, manufacturing, commissioning and operation of the equipment. It means that verifications and tests must be performed in each project step, and generate clear and impartial documents, which are known as: DQ, FAT, IQ and OQ. All those qualification protocols are part of our standard validation plan.
DQ (Design Qualification)
The DQ document generation is directly linked to the client URS (User Requirement Specifications). A proper analysis of the URS allows to find the differences with the offered solutions. Then a traceability matrix is performed to match URS and Manufacturer offer, in order to elaborate the FDS document (Functional and Design Specifications). This document is part of the DQ, and is the starting point throughout development and manufacturing of the sterilization equipment.
The DQ protocol is a qualification document that has to verify and ensure that the proposed design is in agreement with the customer requirements and legal & safety standards. The DQ should also reflect all the important points to fulfill in the applied standards (EN1422, ISO 11135, 2014/34/UE, 21 CFR Parte 11, etc.).
FAT (Factory Acceptance Tests) – Commissioning
To guarantee the proper operation of the sterilizer, the equipment is assembled in real conditions in the manufacturer building. Once the mechanical and electrical works have been performed, the FAT tests can be performed, which consist in carrying out a series of tests called quality protocols. Among several protocols, it should be checked the installed materials, the safety devices, instrumentation calibration, water tightness of the autoclave, jacket, vaporizer, temperature and humidity uniformity in the chamber and on the internal chamber surfaces, software specifications, etc.
Once everything has been verified, we can issue the corresponding compliance document and the CE marking can be installed on the sterilizer.
IQ (Installation Qualification) & OQ (Operation Qualification)
If the customer accepts the FAT document (explained above), then the chamber can be sent to their facilities, and the mechanical and electrical installation can start. So the control tests can begin, before starting with the IQ (Installation qualification). We check through the documents that the sterilization chamber and ancillary equipment (catalytic system, pre-conditioning cells, vacuum pump skids...) fulfill the design specifications (DQ) and the applicable safety regulations. Some FAT tests should be repeated as well, because the equipment uses to be dismounted for the transport. Utilities are also checked (water, steam, N2, chilled water, hot water, electricity ...) and they have to meet with the characteristics defined at beginning of the project (rates, connections, pressures ...).
So once the IQ is performed, OQ can be executed. The goal of the operation qualification is to check the proper functioning of the overall installation, in the client facilities. In these protocols (non-exhaustive list), the leak tests of chamber and jacket as well as piping, thermal mapping, vacuum test, control system (alarms, screens, recipes, etc.).
It is important to highlight that during the last years, IQ/OQ tend to replace the former SAT (Site Acceptance Tests). Now, the final users like to use the IQ/OQ protocols instead of SAT, as they are much more detailed and accurate to qualify the installation.
PQ (Performance Qualification)
An additional support can be offered to the manufacturer Company, which is the PQ (Performance Qualification). It is a support that some companies require due to their lack of knowledge in EtO sterilization.
The knowledge of the EO sterilization process, together with a deep experience on the products to sterilize, lead to the development of an efficient PQ validation plan, ensuring its success. The PQ validation allows to establish ideal parameters for the recipe and to ensure an efficient and reliable sterilization cycle.
Software validation Protocols – SDS, SMDS, SMTS
The ETO sterilization chambers are commissioned with a control system that allows to the user to perform the equipment operations in a safe way, and following the risk analysis that has been issued at the beginning of each project. The software has an important role in EtO sterilization equipment because of the risks this sterilization method supposes. Don’t forget that ETO gas is carcinogenic, explosive, flammable, toxic and for these reasons very strict safety steps should be involved from the very beginning to the employees and the sterilization process.
The correct sterilizer operations have to be validated through software validation protocols:
- SDS (Software and Design Specifications).
- SMDS (Software Module Design Specifications).
- SMTS (Software Module Test Specifications).
These specifications pretend to ensure that the risk analysis, as well as the GAMP aspects, have been fulfilled. Complying with all these meticulous protocols, the client is claimed of buying a safe and reliable equipment with the appropriate documentation for future audits.
A perfect foresight of the project is substantial, in addition to defining a validation plan to indicate tests and obtained results after the execution of the qualifications and ethylene oxide sterilization protocols, and they must be transparent.
It is essential to entrust on professionals, specialized in ethylene oxide sterilization process, to use customized autoclaves that are 100% adapted to the user needs. It is the success key to have an EtO sterilization facility running well.
As expert, RSD team is well qualified to deliver demanding qualification and validation package, adapted to EO sterilization equipment.
Besides turnkey sterilization solution, we also offer a large game of EtO sterilization services.
Don’t hesitate to contact us for any enquiry.
EtO sterilization equipment Qualification & Validation
