A Ethylene Oxide sterilization equipment upgrade
An EO sterilization facility upgrade, to meet the ATEX regulations
The ATEX regulation is essential in the ethylene oxide sterilization plant due to the risk of a potential explosive atmosphere. The latest ATEX regulation updates have happened in 2016 and 2018. It is important meet this directive to have a facility as safe as possible.
To do those upgrade works, it is a must to trust in a competent company having the Ism-Atex certificate for its staff, and that have a wide ethylene oxide sterilization knowledge on the process.
RSD Staff has ISM-ATEX certified
 All the members of our team are Ism-ATEX certified by the INERIS notified body. When a sterilization installation needs to be upgraded, we start first with an ATEX audit to detect any potential aspect that could not respect the ATEX regulation, taking into account ATEX classifications, analyzing protection modes, checking ATEX certificates of the components, inspecting the electrical installation, ….
All the members of our team are Ism-ATEX certified by the INERIS notified body. When a sterilization installation needs to be upgraded, we start first with an ATEX audit to detect any potential aspect that could not respect the ATEX regulation, taking into account ATEX classifications, analyzing protection modes, checking ATEX certificates of the components, inspecting the electrical installation, ….
Then a report is delivered to the customer, in which we identify and indicate all the points and anomalies that don´t respect current ATEX regulation, including the solutions and possible modifications to be implemented. Once the Audit is performed, we propose to take care of the required modifications.
Installation upgrades, sometimes necessary to improve the productivity and the efficiency
The demands and the challenges that is facing the pharmaceutical field is more and more important and complex. For this reason, they have led to promote the existing facility upgrades to use the highest technologies, always based on a safe process and equipment.
RSD, your EtO equipment manufacturer

We control the whole process and we’ll be able to propose you any improvement that can be made in your sterilizer, different skids and ancillaries equipment that are part of the sterilization plant as per: steam generator, vacuum pump skid, vaporizer skid, ….
First, we analyze the customer’s URS (user requirement specifications) and we perform a safety audit of the sterilization facility. Once this step is done, we can propose a series of modifications that can be carried out to the installation, first to fulfil the mandatory regulations in terms of safety aspects, but also to implement possible mechanical, electrical or software improvements to increase efficiency and productivity.
In some particular cases, it’s worth buying a new sterilization equipment instead of trying to refurbish an existing one, especially if the installation is very old.
RSD not only designs, manufactures, qualifies and commissions ethylene oxide sterilization chambers, but also takes care of any other equipment that are part of a comprehensive EtO installation. Exhaustive quality protocols are provided, including their execution to deliver a qualified and validated sterilization installation.
Control system upgrade (SCADA)
The control system is a key element to ensure a safe and optimum EtO sterilization process. It has to be adapted to every customer’s requirements and needs, and should be able to deliver the safest solution for the process.
RSD proposes its own control system for SCADA, ONYX
Apart the fact that RSD uses as standard a safety PLC (SIL2, according to IEC 61508) where all the information to manage the cycle in the safest conditions, it has been developed a SCADA control system - ONYX that gives many possibilities in terms of cycle reports, usernames and passwords, graphs to see analogical and digital signals, cycle recipes, automatic / semi-automatic operations, among others.
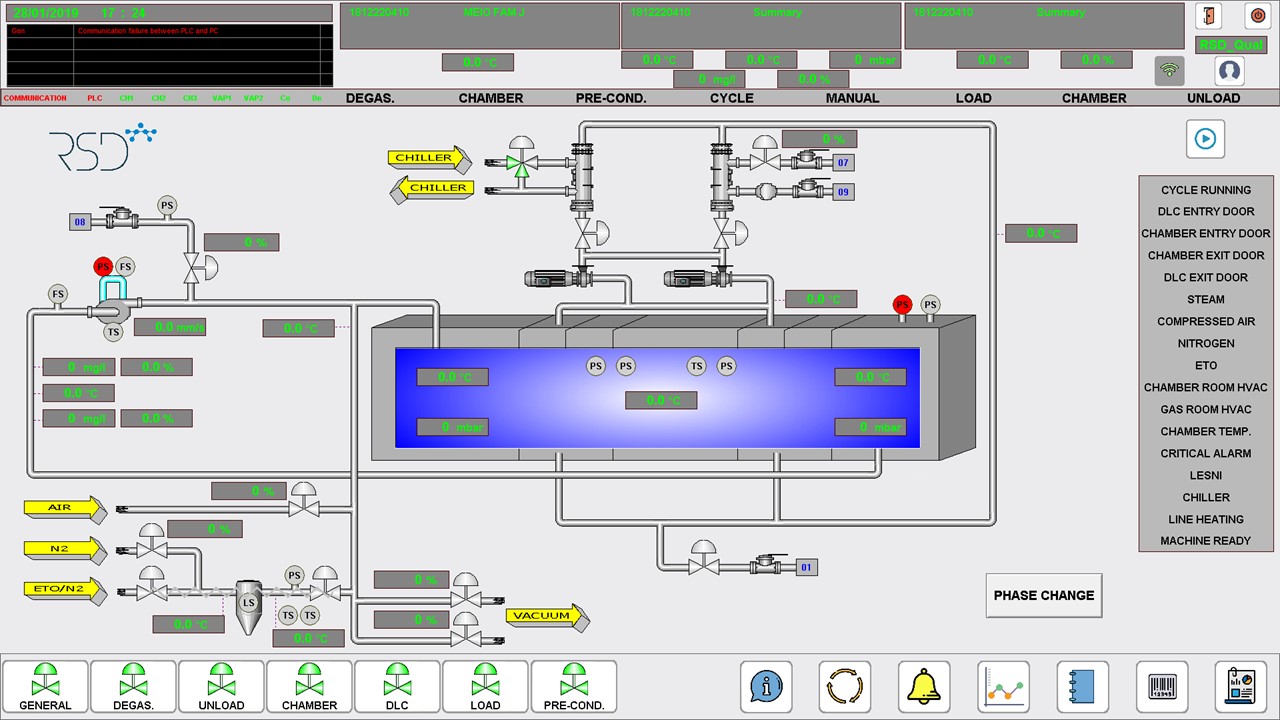 Our ONYX control system allows:
Our ONYX control system allows:
- To record all the steps of the process (pre-conditioning, sterilization, aeration, gas room) in order to don't lose any data, offering full reports with the necessary information.
- To design flexible and safe cycles. The customers can design their own cycles with a high level of safety and flexibility.
- The automatic revision of the inflammable triangle before saving a recipe. If the cycle is potentially dangerous, the control system does not allow to launch it.
- Independent Monitoring System can be installed to those customers that want to separate the record of some critical parameters.
- To operate in manual or automatic modes
- ONYX control system is programmed according to our risk analysis & HAZOP to ensure safe operations. It meets the strictest normatives and guidelines like GAMP5 and FDA 21 CFR Part 11.
- …
Most of those installation upgrades are complex and hard to perform, and a maximum of precautions should be taken. A team of EO sterilization specialists and experts should be in charge of doing those complicated tasks, to avoid any risk for the installation and especially for the personal. RSD, specializing in EtO sterilization process and considered as one of the European leader for EO sterilizer manufacturing, offers its services to upgrade your EtO sterilization installation.
Further information about our ethylene oxide sterilization services, contact-us .
EtO sterilizersEtO process expertise ONYX control system
