Due to its particular characteristics, the ethylene oxide sterilization is a process that requires a specific know-how, and a wide experience in this field is essential for the understanding of the customer needs to offer them the most suitable solutions.
A comprehensive ETO sterilization plant is a major project. Indeed, an ETO sterilization facility is composed of one or several industrial autoclaves with their ancillary equipment that must be properly designed, manufactured and commissioning. The knowledge and control of every steps of project are indispensable to ensure safe and reliable operations.
RSD, specialist in industrial sterilization, is an important partner as ethylene oxide sterilizer manufacturer for pharmaceutical and medical device industries.
Below, we explain you briefly our comprehensive ETO sterilization solutions and how we manage these types of projects. We also offer our services to deliver an efficient, safe and tailored-made installation.
Our ETO sterilization solution
 When we deal with comprehensive ethylene oxide sterilization plant, it is not only the industrial autoclave. All the following items use to be included:
When we deal with comprehensive ethylene oxide sterilization plant, it is not only the industrial autoclave. All the following items use to be included:
- Preconditioning cells
- Degassing cells
- Gas treatment system: Catalytic system or Scrubber
- Steam generator
- Vacuum pump
It is a major project that is achieved in which the design, the installation and the qualification have to be correctly executed.
RSD manufactures and installs the chamber as well as all those ancillary equipment as part of this industrial sterilization plant, in accordance with ATEX regulation and GMP guidelines. We propose comprehensive solutions that are totally adapted to the customer requirements in order to deliver an optimal turnkey project.
Engineering works of an ETO sterilization project
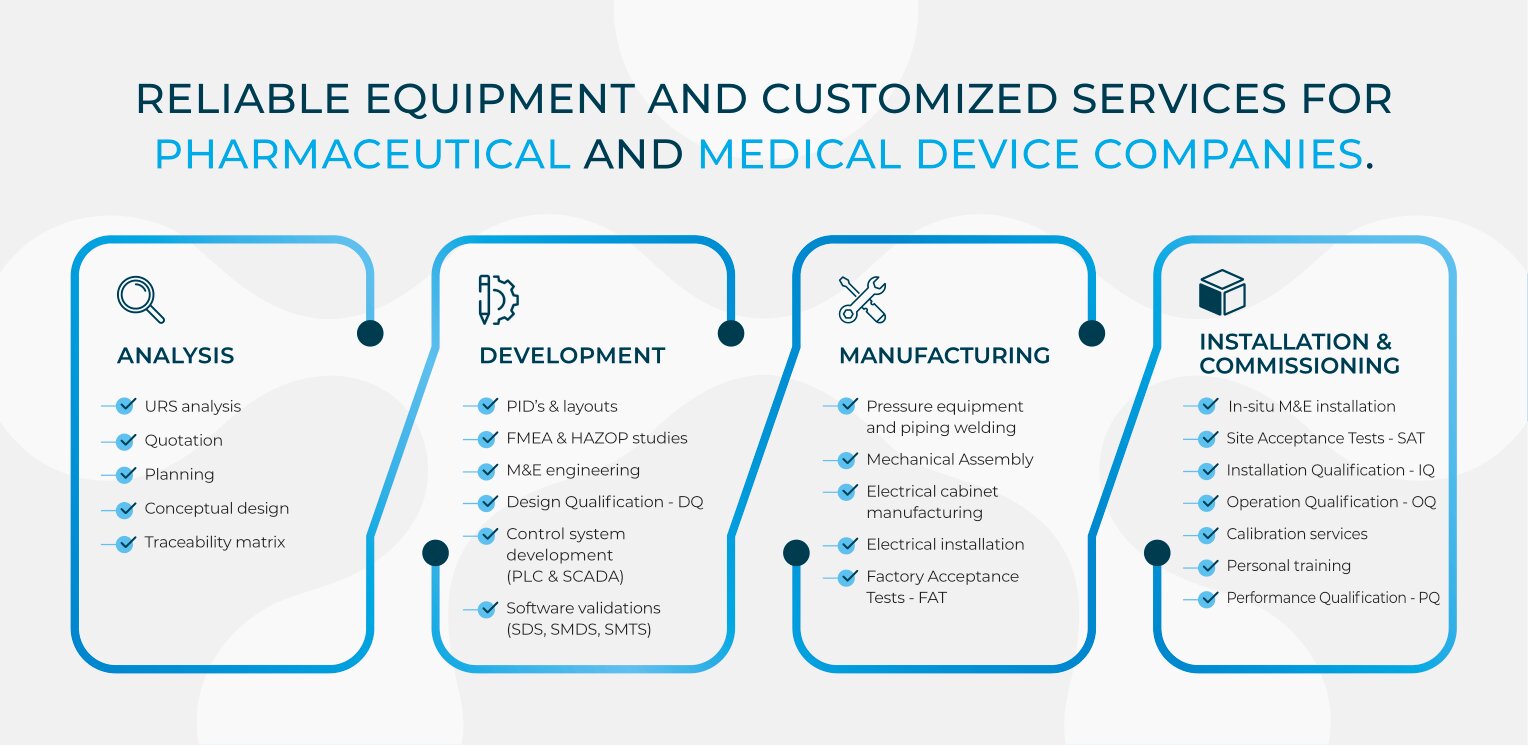
To ensure the success of the project, it is important to understand and to take into account all the requirements and needs of the final user, the customer. For this reason, in RSD, we pay special attention to the URS (User Requirement Specifications) to define the technical aspects of each project, in order to suit the equipment to the type of products to sterilize. Once all the preliminary studies done, we perform a deep risk analysis before starting with the mechanical & electrical engineering using 3D-CAD tools, that allows us to take care of every single detail of the installation. Then our control team is in charge of the development of the PLC and SCADA programming, according to the HAZOP / FMEA, to ensure proper and safe operations of the equipment and the process.
Any test, qualification and validation during the equipment commissioning is under our responsibility. In addition, we generate and execute an exhaustive list of strict quality protocols for:
- DQ Design Qualification
- FDS Functional and Design Specifications
- SDS Software Design Specifications
- SMDS Software Module & Design Specifications
- SMTS Software Module & Test Specifications
- FAT Factory Acceptance Test
- IQ Installation Qualification
- OQ Operation Qualification
Because of the fact that we handle the project from the beginning to the end, we are the unique intermediary with the customer. We give comprehensive solutions for design, process, control and ethylene oxide sterilization validation. It is important for us to establish long term relationships with our customers, and to work hand in hand with them.
Advantages of ETO sterilization plant design by RSD
RSD is in charge of the development, manufacturing, qualification and commissioning of sterilization installations. In addition, our commitment is to propose a safe, efficient and productive installations to satisfy 100% the customers’ expectations.
Entrusting the projects to RSD, means to take advantage of the following benefits:
- Expert in EO sterilization: expertise and qualified staff.
- Turnkey EO sterilization system: comprehensive solution for ETO sterilization.
- Customized industrial equipment: we design and manufacture industrial sterilizers from 4 pallets. We adapt them to the customer’s requirements and type of products to sterilize….
- State-of-the-art components to ensure long term viability of the equipment.
- Modular design to ensure the safety and the efficacy of the process.
- Qualification and commissioning of the equipment (including FAT, DQ, IQ, OQ).
- PLC and SCADA control system to provide high process performance.
- Support and advices during the project.
ETO sterilization services
Specializing in industrial sterilization, and especially in ethylene oxide sterilization, we offer a wide range of sterilization services, in addition to sterilizer manufacturing and turnkey projects.
They are customized and high quality services focused on ETO sterilization process and ETO equipment.
- EO Cycle optimization and validation;
- Maintenance of sterilizers / sterilization units;
- Installation and verification services;
- Protocol generation and performance (FDS, DQ, FAT, IQ, OQ and PQ);
- EO sterilization plant sizing, according to customer requirements;
- Utility calculations (N2/CO2, EO, chilled water, steam,…), needed for the running of a EO sterilization installation;
- Safety audit to fulfill the ATEX 2014/34/UE directive;
- Existing installation upgrade to be conformed to ATEX directive;
- Parametrical release implantation on existing sterilizers;
- Flammability calculations;
- ROI calculations for a sterilization project investment;
- Expertise and advices for technological, strategic and operative problems;
- …
Our qualified employees have the Ism-ATEX certificated, level 1 and level 2, for design and maintenance of explosive atmosphere installations, highly recommended for a comprehensive ethylene oxide sterilization facility.
For further information, do not hesitate to contact us. We will be pleased to help you.
EtO sterilizers EtO process expertise
